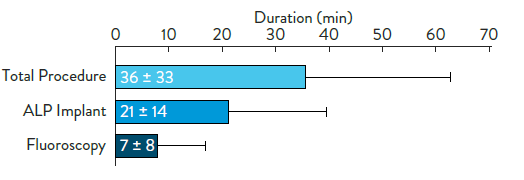
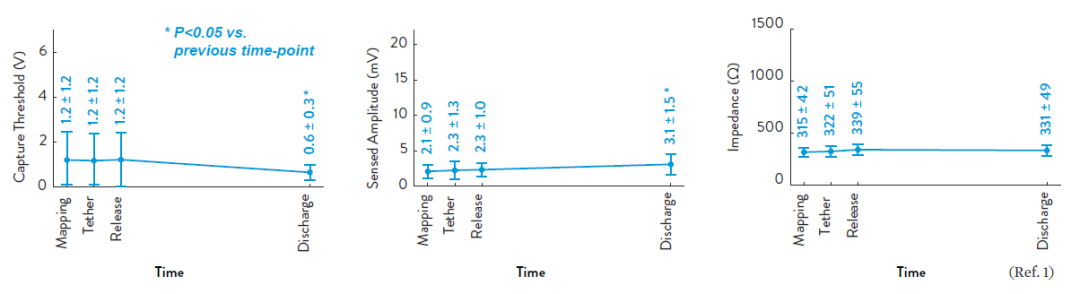
1. Tjong FVY, Reddy VY. Permanent leadless cardiac pacemaker therapy:
a comprehensive review. Circulation. 2017;135(15):1458–70.
https:// doi. org/ 10. 1161/ CIRCU LATIO NAHA. 116. 025037.
2. Sattar Y, et al. Complications of leadless vs conventional (lead)
artificial pacemakers – a retrospective review. Journal of Community
Hospital Internal Medicine Perspectives. 2020;10(4):328–33.
https:// doi. org/ 10. 1080/ 20009 666. 2020. 17869 01.
3. Knops RE, et al. A dual-chamber leadless pacemaker. N Engl J
Med. 2023. https:// doi. org/ 10. 1056/ NEJMo a2300 080.
4. Ip JE, et al. Atrioventricular synchrony delivered by a dual-chamber
leadless pacemaker system. Circulation. 2024. https:// doi. org/
10. 1161/ CIRCU LATIO NAHA. 124. 069006.
5. Hindricks G, et al. Six-month electrical performance of the first
dual-chamber leadless pacemaker. Heart Rhythm. 2024. https://
doi. org/ 10. 1016/j. hrthm. 2024. 04. 091.
6. Banker RS, et al. Retrieval of chronically implanted dual-chamber leadless
pacemakers in an ovine model. Circ Arrhythm Electrophysiol.
2023;16(10):e012232. https:// doi. org/ 10. 1161/ CIRCEP. 123. 012232.
7. Rashtian M, et al. Preclinical safety and electrical performance
of novel atrial leadless pacemaker with dual-helix fixation. Heart
Rhythm. 2022;19(5):776–81. https:// doi. org/ 10. 1016/j. hrthm.
2022. 01. 021.
8. Nielsen JC, et al. A comparison of single-lead atrial pacing
with dual-chamber pacing in sick sinus syndrome. Eur Heart J.
2011;32(6):686–96. https:// doi. org/ 10. 1093/ eurhe artj/ ehr022.
9. Nadeem B, et al. Outcomes of concurrent and delayed leadless
pacemaker implantation following extraction of infected cardiovascular
implantable electronic device. J Interv Card Electrophysiol.
2024. https:// doi. org/ 10. 1007/ s10840- 024- 01960-2.
10. Reddy VY, et al. 1-Year outcomes of a leadless ventricular pacemaker:
the LEADLESS II (phase 2) trial. JACC Clin Electrophysiol.
2023;9(7 Pt 2):1187–9. https:// doi. org/ 10. 1016/j. jacep.
2023. 01. 031.
11. Reddy VY, et al. Primary results on safety and efficacy from the
LEADLESS II-phase 2 worldwide clinical trial. JACC Clin Electrophysiol.
2022;8(1):115–7. https:// doi. org/ 10. 1016/j. jacep. 2021. 11. 002.
12. Sundaram S, Alyesh D, Walker L, Zipse MM. The 1(st) implantation
of an atrial only leadless pacemaker in right atrial appendage.
J Interv Card Electrophysiol. 2023;66(9):1955–8. https:// doi. org/
10. 1007/ s10840- 023- 01644-3.
13. Ferrick AM. Vasovagal syncope successfully treated with an atrial
leadless pacemaker. HeartRhythm Case Reports. 2024;10(9):691–
2. https:// doi. org/ 10. 1016/j. hrcr. 2024. 08. 011.
14. Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo
Z, Sadr-Ameli MA. Predictors of venous obstruction following
pacemaker or implantable cardioverter-defibrillator implantation:
a contrast venographic study on 100 patients admitted for
generator change, lead revision, or device upgrade. Europace.
2007;9(5):328–32. https:// doi. org/ 10. 1093/ europ ace/ eum019.
15. Zimetbaum P, Carroll BJ, Locke AH, Secemsky E, Schermerhorn
M. Lead-related venous obstruction in patients with implanted cardiac
devices: JACC review topic of the week. J Am Coll Cardiol.
2022;79(3):299–308. https:// doi. org/ 10. 1016/j. jacc. 2021. 11. 017.
16. Nowak B, Misselwitz B, I. o. Q. A. H. Expert Committee Pacemaker.
Effects of increasing age onto procedural parameters in
pacemaker implantation: results of an obligatory external quality
control program. Europace. 2009;11(1):75–9. https:// doi. org/ 10.
1093/ europ ace/ eun293.
17. Nair DG, et al. Early real-world implant experience with a helixfixation
ventricular leadless pacemaker. J Interv Card Electrophysiol.
2024. https:// doi. org/ 10. 1007/ s10840- 024- 01791-1.
18. Roberts PR, et al. A leadless pacemaker in the real-world setting:
the Micra Transcatheter Pacing System Post-Approval Registry.
Heart Rhythm. 2017;14(9):1375–9. https:// doi. org/ 10. 1016/j.
hrthm. 2017. 05. 017.
19. El-Chami MF, et al. Leadless pacemakers at 5-year follow-up: the
Micra transcatheter pacing system post-approval registry. Eur Heart
J. 2024;45(14):1241–51. https:// doi. org/ 10. 1093/ eurhe artj/ ehae1 01.
20. Garweg C, et al. A leadless ventricular pacemaker providing
atrioventricular synchronous pacing in the real-world setting:
12-month results from the Micra AV post-approval registry. Heart
Rhythm. 2024. https:// doi. org/ 10. 1016/j. hrthm. 2024. 06. 008.
Publisher's Note Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations.