二尖瓣反流介入治疗解决方案再添新!近日,由成都赛拉诺医疗科技股份有限公司自主创新研发的新型经导管二尖瓣瓣环修复系统Silara MiBridge™首例人体应用6个月临床随访结果在TVT 2023(经导管瓣膜治疗结构性心脏病峰会)发布。该系统的成功手术和令人欣喜的临床疗效彰显了成都赛拉诺强大的研发创新能力,标志着成都赛拉诺成功布局二尖瓣介入治疗产品,是公司“深耕结构性心脏病治疗领域”的实践证明!
The Silara MiBridge™, a new Transcatheter Mitral Valve Repair (TMVr) device designed by Silara Medtech was highlighted at TVT 2023, on June 7-10 in Phoenix, AZ. The Silara MiBridge™, a next generation of direct annuloplasty system, is designed to address limitations of previous annuloplasty devices. The debut of Silara MiBridge™ is a milestone event for Silara Medtech, highlighting the company’s strong R&D and innovation capabilities, successfully expanding its product pipeline for structural heart disease.
2023年6月8日,在美国菲尼克斯召开的TVT 2023国际大会上,来自UC DAVIS心血管中心的Gagan D. Singh教授公布了由赛拉诺医疗研发的新型经导管二尖瓣瓣环修复系统Silara MiBridge™首例人体应用6个月随访结果。该患者70岁,入院时二尖瓣反流程度3+,心功能 III级,合并高血压、房颤及三尖瓣反流。经过心脏团队对病例的充分评估,决定采用Silara MiBridge™经导管二尖瓣修复系统进行手术。最终,该患者获得有效治疗,术后二尖瓣反流程度由中重度降至微量,压差1mmHg,心功能 I 级,临床症状明显改善。6 个月随访时,二尖瓣反流程度稳定保持在微量,血流动力学保持良好。
The procedure had promising clinical outcomes. On June 8, 2023 Prof. Gagan D. Singh, director of Clinical Cardiovascular Research Unit, UC DAVIS presented the results of the first-in-human case treated by Silara MiBridge™ in a 70-year-old patient. The patient has MR 3+ (NYHA Class III) with hypertension, atrial fibrillation and tricuspid regurgitation. After the procedure the MR was reduced from moderate/severe to trace with a gradient of 1mmHg. At 6-month follow-up, TTE showed only trace MR and the device remained stable. Meanwhile, patient’s cardiac function has improved from NYHA Class III to Class I. The procedure demonstrated initial feasibility of the Silara MiBridge™ system in MR treatment.
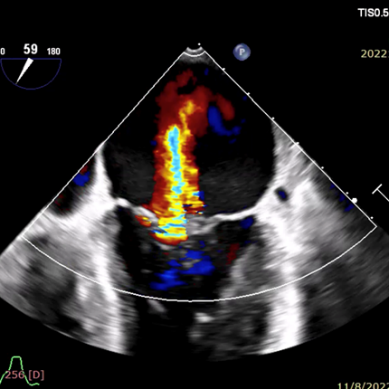
术前超声
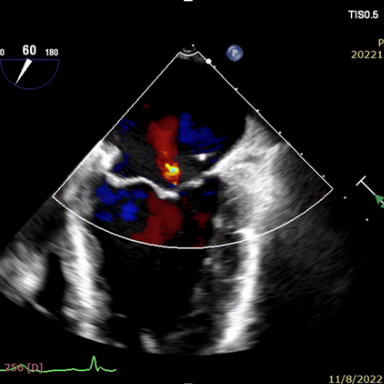
术后即刻
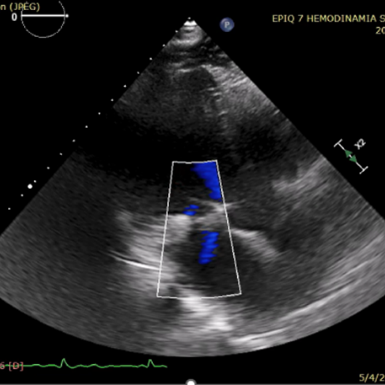
6个月随访
二尖瓣疾病是常见的心脏瓣膜病,且以二尖瓣反流为主。据Hu P等的一项调查研究,中国35岁及以上人群中的中重度二尖瓣反流总体发病率为1.2%[1],且随年龄增长不断升高。一直以来,中重度二尖瓣反流的介入治疗都是心血管领域的难点和热点,主要可分为经导管二尖瓣修复(TMVr)和经导管二尖瓣置换(TMVR)两大类。其中,经导管二尖瓣修复又可大致分为缘对缘修复(TEER)、瓣环成形术和腱索修复等不同术式。本次亮相TVT的Silara MiBridge™经导管二尖瓣修复系统属于瓣环成形术中的一种。手术时分别在二尖瓣前叶(A区)、后叶(P区)瓣环处植入三个分散式组件,通过对该组件的不断调整直接减小A-P距离,从而使前瓣叶和后瓣叶对合良好,最终达到二尖瓣无反流或减轻至微量反流的治疗目的。Silara MiBridge™的创新设计,让手术操作简便且可实现对瓣叶不同区域的距离调整,使得该产品只需一个型号就可适应绝大多数情况下的二尖瓣解剖结构。其首例人体的成功应用令人振奋和期待,未来必将为众多二尖瓣反流患者提供全新的治疗选择。
The overall incidence of moderate to severe MR in the population of 35y and older in China has been reported to be 1.2%[1]. Patients with MR who are at high risk or contraindicated surgery are potential candidates for transcatheter intervention. The Silara MiBridge™ system is designed to directly reduce anteroposterior (A-P) MV annular dimensions, thereby improving leaflet coaptation. The three separate components of the device can be adapted to various anatomy and the design allows for real-time adjusting of annular dimension. Silara MiBridge™ is an innovative device that should benefit patients suffering from MR.
关于赛拉诺医疗
About Silara Medtech
成都赛拉诺医疗科技股份有限公司成立于2017年,是一家立足本土、拥有全球化视野的创新型企业。公司总部位于中国成都,在美国加州设立100%控股子公司Silara Medtech Inc. 。中美两地均已获得BSI颁发的ISO13485:2016质量管理体系认证证书。依托中美双核研发机制,公司将持续聚焦国际医学前沿,保持源源不断的创新活力,以期为临床医生和患者提供安全有效的高品质医疗器械,为生命健康护航。
Silara Medtech is an innovative company dedicated to developing novel therapies for structural heart disease. It’s headquartered in Chengdu, China, and has a wholly-owned subsidiary in California, USA. Both facilities in China and USA have been accredited by BSI to ISO 13485:2016. Silara Medtech will maintain the vitality and enthusiasm for innovation, and continue to commit itself to providing integrated solutions for physicians and patients.
参考文献:
[1] Hu P, Liu XB, Liang J, et al. A hospital-based survey of patients with severe valvular heart disease in China. Int J Cardiol. 2017 Mar 15.