心房颤动导管消融相关无症状脑梗塞
2015-08-11 15:22
心房颤动(房颤)发生血栓栓塞的风险比正常人群增加5-7倍,临床上约20%脑卒中由房颤引发[1]。近年来房颤导管消融术日臻成熟,显著提高窦性心律的转复及维持,降低血栓栓塞的发生风险,已成为房颤重要的有效治疗方案[2-6]。房颤导管消融术可以降低脑栓塞发生风险,但这一结论主要基于临床症状性脑梗塞。近年来越来越多的研究[7-9]发现,房颤导管消融增加了无症状脑梗塞的发生,这使得房颤的导管消融的临床获益面临质疑。本文就相关情况简要介绍如下。
1 定义
房颤导管消融患者术前24小时及术后24小时内行场强1.5T以上的MRI检查,包括弥散加权(diffusion-weighted, DWI)及水抑制反转成像加权序列(without fluid attenuated inverse recovery sequence, FLAIR),来筛查术后新发脑梗死病灶(图1[7])。如MRI显示阳性病灶,而患者无相关临床症状,查体无神经系统定位体征,即定义为房颤导管消融相关的无症状脑梗塞[7]。比较性研究发现,CT检查不易发现微小脑梗塞病灶,且缺血性脑损伤早期DWI敏感性更高,对微小脑梗塞灶的检出率是FLAIR的3倍,因此MRI-DWI是诊断无症状脑梗塞金标准[10]。研究显示无症状性脑梗塞的最佳检查时间窗是消融术后24-72小时,实践通常在术后2-7天进行筛查[11]。
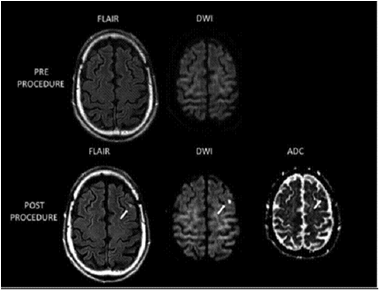
图1 房颤导管消融术后MRI新发脑梗塞灶 上图:术前,下图:术后
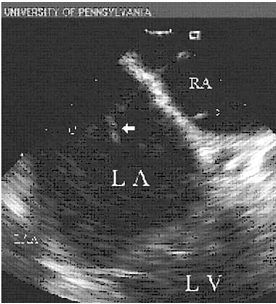
图2. 心脏内超声探及房颤导管消融过程中左房内附壁于电极导管的血栓
2 流行病学
由于相关临床研究的规模各异,且采用的消融策略、技术参数和选择的检查时间窗不同,消融术后无症状脑梗塞的发生率不尽相同。研究入选的患者数量早在1999年Zhou L[12]等开始注意到射频导管消融增加血栓栓塞的发生,Medline近10年的相关研究文献荟萃显示,快速性心律失常射频消融术后血栓栓塞并发症的整体发生率约为0.6%,房颤迷宫术扩展的线性消融和左室室性心动过速消融时栓塞发生率则高达1.8%-2.0%和2.8%,即使消融过程中应用静脉肝素或调整温度模式,也不会降低此类栓塞风险。2004年Ren等[13]在232名房颤患者消融术中采用心腔内超声(intracardiac echocardiography,ICE),检出24例左房血栓(10.3%),而且心房血栓多附着于鞘管和标测电极(见图2[13])。2006年Lickfett L[14]等率先应用DWI-MRI检查,研究房颤导管消融患者无症状脑梗塞的发生情况。入选的20例房颤患者均接受盐水灌注导管行肺静脉电隔离,术前、术后分别进行头部DWI-MRI检查,所有患者均无脑梗塞相关症状和体征,影像学检查提示2名患者出现新发脑梗塞灶(10%)。该研究引起人们广泛关注,并对“房颤导管消融能降低脑栓塞风险”提出了质疑。此后报道了一系列房颤导管消融伴发的无症状脑梗塞病例,显示消融术后无症状发生率在7%-38%[15-17]。2010年Gaita F[7]等报道了房颤射频消融多种术式其无症状脑梗塞发生的前瞻性研究,入选232例患者,基础术式为肺静脉电隔离,部分加线性消融或碎裂电位消融。结果发现术后症状性脑血管事件发生率仅为0.4%,而经DWI-MRI检查确诊的无症状脑梗塞发生率高达14%。该研究还对术中药物及电复律患者进行了分析,复律患者共61例,其中15例发生无症状脑梗塞,占新发无症状脑梗塞总数的患者45%(15/33);另46例未发生无症状脑梗塞,占未发生脑梗塞人群的23%(46/199),P < 0.009,提示术中复律是无症状脑梗塞发生的独立危险因素。术中复律治疗增加无症状脑梗塞的发生,其机制可能是复律过程中增加了左心耳内微小血栓脱落的机率。
房颤消融过程中超声多普勒探查微血栓信号(microembolic signals, MES)也开始应用于无症状脑梗塞的研究。消融时心腔内微气泡形成及MES均可能引起无症状脑梗塞发生,有待更多的研究证实。2006年Kilicaslan F[18]等研究了房颤导管消融过程中心腔内微气泡数量与脑血管MES的关系。该研究入组202例患者,在消融时应用ICE探测心腔内微气泡形成数量,随后通过经颅多普勒探测大脑中动脉MES,发现90%的MES发生在左房消融过程中,超过 85%的MES发生在微气泡形成之后,显示MES与微气泡的形成直接相关,而发生脑栓塞事件的患者MES数量显著增多。2014年Nagy-Balo[19]等报道了射频消融生物物理参数对MES形成的影响。该研究入选了48例患者,应用环形多极电极行肺静脉隔离,分析了834次射频消融参数,结果显示导管与组织接触不良、温度较低(45℃–55℃)或较高(高于62℃)、输出能量高、消融电极数目多等均可导致大脑中动脉MES 计数增加。
3 发生机制
房颤消融引发脑栓塞可能机制包括[10,11]: ①导管操作 :鞘内血栓脱落及操作过程中气体栓塞;导管插入心腔也可引起凝血酶原III水平增加,诱发血栓栓塞;②消融:导管消融引起组织的内皮损伤,电学透壁损伤,局部对流加热进一步激活血小板和凝血酶原;消融过程中微气泡形成[20];③复律:房颤电复律过程中机械损伤,及恢复心房正常收缩后,可引起左房微小血栓脱落引发脑栓塞;④其它 :不同术式、不同消融能源、能量大小、消融导管类型亦对左房血栓形成可能有一定影响。
4 围手术期处理及预防策略
4.1 围手术期抗凝策略
房颤诊疗指南建议房颤射频消融手术术前通常停用华法林2-3天,替换为低分子肝素皮下注射。术中应用肝素抗凝,要求活化凝血时间(ACT)目标值>250s,术后桥接华法林抗凝治疗。但部分研究显示目前的抗凝策略不能有效预防血栓栓塞风险。
ACT是围手术期血栓栓塞独立危险预测因素,术中ACT时间长短可能直接影响血栓的形成。Ren等[21]房颤射频消融术中抗凝研究共入选511例患者,分为两组。应用心腔内超声发现ACT 250-300s组左房血栓发生率11.2% (33/294) ,而ACT>300s组左房血栓发生率仅为2.8% (6/217 ) ,P < 0.05,提示加强消融过程中抗凝强度可减少血栓发生,均无症状性缺血性卒中事件。
由于目前抗凝策略术前中断华法林,低分子肝素替代治疗,术后继续应用华法林,需要数天才能达到有效抗凝强度,从而增加了血栓栓塞风险。2010年Hussein[22]等在3052例房颤导管消融患者未采用桥接治疗,围手术期继续华法林抗凝,INR平均2.52±0.62,结果缺血性卒中3例,出血性卒中1例,不严重出血34例(1.11%),提示围手术期持续应用华法林安全有效,显著降低血栓栓塞风险。2014年Biase [23]等进行的一项前瞻性多中心研究评估了房颤导管消融围手术期持续应用华法林血栓栓塞的风险。应用华法林组无症状性脑卒中发生率2.1%,停用华法林组无症状性脑卒中发生率高达14.2%,两组间发生率讯在显著差异(P<0.0001)。可见,中断华法林使围手术期血栓风险明显增加(OR:3.8,95% CI),而围手术期持续应用华法林可明显降低缺血性卒中风险。术后是否继续坚持服用华法林目前尚存在争议,一般如无复发,应用华法林至术后2-3个月。
上述研究提示我们房颤导管消融过程中需加强抗凝以减少血栓栓塞发生风险,术中ACT监测保持在300~350s;术前应用华法林者,术中继续应用;术后坚持服用华法林,INR2~3, 如无复发,应用华法林2~3个月。
4.2 导管操作及术式选择策略
心腔内导管操作应轻柔小心,避免鞘内形成血栓、气体进入、充分回抽及持续肝素盐水冲洗。血栓的形成与消融方法及手术中左房内放置导管数量多少有一定关系,即导管数量越多形成血栓栓塞的机会可能就越多。2011年Gaita[24]等一项前瞻性研究连续入选108例行房颤导管消融患者,等数量分为冷冻球囊导管组、盐水灌注导管组和多电极导管组,均对肺静脉进行电隔离,发现无症状脑梗塞比例分别为:5.6%,8.3%,38.9%。显示不同手术器械对房颤导管消融相关无症状脑梗塞有一定关系,并且左房内导管数目越多,形成血栓栓塞的几率在增加。2014年Gui-jian L等研究[25]发现冷冻球囊,盐水灌注导管,激光球囊,多电极导管和nMARQTM(环形多极消融导管)无症状脑梗塞发生率分别为12.5%,13.0%, 17.3%, 27.6%和 32.6%。上述研究显示单一的冷冻球囊消融发生血栓栓塞风险最低,冷盐水灌注导管消融法需要额外应用Lasso环形电极,因此增加了血栓风险,而多电极导管技术血栓栓塞风险最高,同时提示导管数目的增多也增加血栓栓塞的发生。因此,手术过程中应合理选择器械,尽量减少不必要导管的放置和使用。
5 预后
虽然脑梗塞是无临床症状的,但是这些损害累积性效应,可能会成为脑血管事件的一个沉重负担,导致痴呆、记忆缺失的增加,认知功能减低[26-28]。以后有发生症状性脑梗塞的可能,或使其发生症状性脑梗塞的病情加重,并增加脑梗塞复发的危险。
6 小结
我们不能忽视房颤导管消融相关无症状脑梗塞的发生。目前抗凝策略不能完全有效预防无症状脑梗塞,强化导管消融围手术期抗凝,如术中ACT监测保持在300~350s,围手术期不间断应用华法林抗凝等。细化房颤消融方案,合理选择手术器械及方法,以减少脑梗塞发生。
参考文献
1. Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation[J]. Circ Arrhythmia Electrophysiol 2010, 1:3:32-38.
2.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010,303(4):333-340.
3. Piccini JP, Lopes RD, Kong MH, et al. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials[J]. Circ Arrhythm Electrophysiol. 2009, 2(6):626-633.
4. Pappone C1, Vicedomini G, Augello G, et al. Radiofrequency catheter ablation and antiarrhythmic drug therapy: a prospective, randomized, 4-year follow-up trial: the APAF study. Circ Arrhythm Electrophysiol. 2011, 4(6):808-814.
5. Wokhlu A, Monahan KH, Hodge DO, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. Am Coll Cardiol. 2010, 55(21):2308-2316.
6.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009, 2:349–361.
7. Gaita F, Caponi D, Pianelli M, et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation, 2010, 122(17): 1667-1673.
8. Scaglione M, Blandino A, Raimondo C, et al. Impact of ablation catheter irrigation design on silent cerebral embolism after radiofrequency catheter ablation of atrial fibrillation: Results from a pilot study. Journal of cardiovascular electrophysiology, 2012, 23(8): 801-805.
9. Deneke T, Shin D I, Balta O, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm, 2011, 8(11): 1705-1711.
10. Deneke T, Jais P, Scaglione M, et al. Silent Cerebral Events/Lesions Related to Atrial Fibrillation Ablation: A Clinical Review. J Cardiovasc Electrophysiol. 2015, 26(4):455-463.
11. Kalantarian S, Ay H, Gollub RL, et al. Association Between Atrial Fibrillation and Silent Cerebral Infarctions: A Systematic Review and Meta-analysisAtrial Fibrillation and Silent Cerebral Infarctions. Ann Intern Med. 2014, 161(9):650-658.
12. Zhou L, Keane D, Reed G, et al. Thromboembolic complications of cardiac radiofrequency catheter ablation: A review of the reported incidence, pathogenesis and current research directions. J Cardiovasc Electrophysiol 1999, 10:611-620.
13. Ren JF, Marchlinski FE, Callans DJ et al. Left atrial thrombus associated with ablation for atrial fibrillation: Identification with intracardiac echocardiography. J Am Coll Cardiol 2004;10:1861-1867
14.Lickfett L, Hackenbroch M, Lewalter T, et al. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation[J]. Cardiovasc Electrophysiol. 2006, 17(1):1-7.
15. Wieczorek M, Lukat M, Hoeltgen R, et al. Investigation into Causes of Abnormal Cerebral MRI Findings Following PVAC Duty‐Cycled, Phased RF Ablation of Atrial Fibrillation[J]. Journal of cardiovascular electrophysiology, 2013, 24(2): 121-128.
16.Rillig A, Meyerfeldt U, Tilz R R, et al. Incidence and long-term follow-up of silent cerebral lesions after pulmonary vein isolation using a remote robotic navigation system as compared with manual ablation[J]. Circulation: Arrhythmia and Electrophysiology, 2012, 5(1): 15-21.
17. Siklódy C H, Deneke T, Hocini M, et al. Incidence of Asymptomatic Intracranial Embolic Events After Pulmonary Vein Isolation Comparison of Different Atrial Fibrillation Ablation Technologies in a Multicenter Study[J]. Journal of the American College of Cardiology, 2011, 58(7): 681-688.
18. Kilicaslan F, Verma A, Saad E, et al. Transcranial Doppler detection of microembolic signals during pulmonary vein antrum isolation: implications for titration of radiofrequency energy. Cardiovasc Electrophysiol. 2006, 17(5):495-501.
19. Nagy-Balo E, Kiss A, Condie C, et al. Predictors of cerebral microembolization during phased radiofrequency ablation of atrial fibrillation: analysis of biophysical parameters from the ablation generator. Heart Rhythm. 2014, 11(6):977-983.
20. Oh S, Kilicaslan F, Zhang Y, et al. Avoiding microbubbles formation during radiofrequency left atrial ablation versus continuous microbubbles formation and standard radiofrequency ablation protocols: comparison of energy profiles and chronic lesion characteristics[J]. Cardiovasc Electrophysiol. 2006,17(1):72-77.
21. Ren JF, Marchlinski FE, Callans DJ, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast [J]. Cardiovasc Electrophysiol. 2005, 16(5):474-477.
22.Hussein AA, Martin DO, Saliba W,et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm. 2009, 6(10):1425-1429.
23. Biase L, Gaita F, Toso E, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014, 11(5):791-798.
24. Gaita F, Leclercq J F, Schumacher B, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon[J]. Journal of cardiovascular electrophysiology, 2011, 22(9): 961-968.
25. Gui-jian L, Wen-qing Z, Xing-gang W, et al. Association between Ablation Technology and Asymptomatic Cerebral Injury Following Atrial Fibrillation Ablation. Pacing Clin Electrophysiol. 2014.
26. Schwarz N, Kuniss M, Nedelmann M , et al. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm. 2010;7(12):1761-1767.
27. Vermeer S, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003, 348:1215-1222.
28. Knecht S, Oelschlager C, Duning T, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008, 29:2125–2132.
阅读数: 1513
1 定义
房颤导管消融患者术前24小时及术后24小时内行场强1.5T以上的MRI检查,包括弥散加权(diffusion-weighted, DWI)及水抑制反转成像加权序列(without fluid attenuated inverse recovery sequence, FLAIR),来筛查术后新发脑梗死病灶(图1[7])。如MRI显示阳性病灶,而患者无相关临床症状,查体无神经系统定位体征,即定义为房颤导管消融相关的无症状脑梗塞[7]。比较性研究发现,CT检查不易发现微小脑梗塞病灶,且缺血性脑损伤早期DWI敏感性更高,对微小脑梗塞灶的检出率是FLAIR的3倍,因此MRI-DWI是诊断无症状脑梗塞金标准[10]。研究显示无症状性脑梗塞的最佳检查时间窗是消融术后24-72小时,实践通常在术后2-7天进行筛查[11]。

图1 房颤导管消融术后MRI新发脑梗塞灶 上图:术前,下图:术后

图2. 心脏内超声探及房颤导管消融过程中左房内附壁于电极导管的血栓
2 流行病学
由于相关临床研究的规模各异,且采用的消融策略、技术参数和选择的检查时间窗不同,消融术后无症状脑梗塞的发生率不尽相同。研究入选的患者数量早在1999年Zhou L[12]等开始注意到射频导管消融增加血栓栓塞的发生,Medline近10年的相关研究文献荟萃显示,快速性心律失常射频消融术后血栓栓塞并发症的整体发生率约为0.6%,房颤迷宫术扩展的线性消融和左室室性心动过速消融时栓塞发生率则高达1.8%-2.0%和2.8%,即使消融过程中应用静脉肝素或调整温度模式,也不会降低此类栓塞风险。2004年Ren等[13]在232名房颤患者消融术中采用心腔内超声(intracardiac echocardiography,ICE),检出24例左房血栓(10.3%),而且心房血栓多附着于鞘管和标测电极(见图2[13])。2006年Lickfett L[14]等率先应用DWI-MRI检查,研究房颤导管消融患者无症状脑梗塞的发生情况。入选的20例房颤患者均接受盐水灌注导管行肺静脉电隔离,术前、术后分别进行头部DWI-MRI检查,所有患者均无脑梗塞相关症状和体征,影像学检查提示2名患者出现新发脑梗塞灶(10%)。该研究引起人们广泛关注,并对“房颤导管消融能降低脑栓塞风险”提出了质疑。此后报道了一系列房颤导管消融伴发的无症状脑梗塞病例,显示消融术后无症状发生率在7%-38%[15-17]。2010年Gaita F[7]等报道了房颤射频消融多种术式其无症状脑梗塞发生的前瞻性研究,入选232例患者,基础术式为肺静脉电隔离,部分加线性消融或碎裂电位消融。结果发现术后症状性脑血管事件发生率仅为0.4%,而经DWI-MRI检查确诊的无症状脑梗塞发生率高达14%。该研究还对术中药物及电复律患者进行了分析,复律患者共61例,其中15例发生无症状脑梗塞,占新发无症状脑梗塞总数的患者45%(15/33);另46例未发生无症状脑梗塞,占未发生脑梗塞人群的23%(46/199),P < 0.009,提示术中复律是无症状脑梗塞发生的独立危险因素。术中复律治疗增加无症状脑梗塞的发生,其机制可能是复律过程中增加了左心耳内微小血栓脱落的机率。
房颤消融过程中超声多普勒探查微血栓信号(microembolic signals, MES)也开始应用于无症状脑梗塞的研究。消融时心腔内微气泡形成及MES均可能引起无症状脑梗塞发生,有待更多的研究证实。2006年Kilicaslan F[18]等研究了房颤导管消融过程中心腔内微气泡数量与脑血管MES的关系。该研究入组202例患者,在消融时应用ICE探测心腔内微气泡形成数量,随后通过经颅多普勒探测大脑中动脉MES,发现90%的MES发生在左房消融过程中,超过 85%的MES发生在微气泡形成之后,显示MES与微气泡的形成直接相关,而发生脑栓塞事件的患者MES数量显著增多。2014年Nagy-Balo[19]等报道了射频消融生物物理参数对MES形成的影响。该研究入选了48例患者,应用环形多极电极行肺静脉隔离,分析了834次射频消融参数,结果显示导管与组织接触不良、温度较低(45℃–55℃)或较高(高于62℃)、输出能量高、消融电极数目多等均可导致大脑中动脉MES 计数增加。
3 发生机制
房颤消融引发脑栓塞可能机制包括[10,11]: ①导管操作 :鞘内血栓脱落及操作过程中气体栓塞;导管插入心腔也可引起凝血酶原III水平增加,诱发血栓栓塞;②消融:导管消融引起组织的内皮损伤,电学透壁损伤,局部对流加热进一步激活血小板和凝血酶原;消融过程中微气泡形成[20];③复律:房颤电复律过程中机械损伤,及恢复心房正常收缩后,可引起左房微小血栓脱落引发脑栓塞;④其它 :不同术式、不同消融能源、能量大小、消融导管类型亦对左房血栓形成可能有一定影响。
4 围手术期处理及预防策略
4.1 围手术期抗凝策略
房颤诊疗指南建议房颤射频消融手术术前通常停用华法林2-3天,替换为低分子肝素皮下注射。术中应用肝素抗凝,要求活化凝血时间(ACT)目标值>250s,术后桥接华法林抗凝治疗。但部分研究显示目前的抗凝策略不能有效预防血栓栓塞风险。
ACT是围手术期血栓栓塞独立危险预测因素,术中ACT时间长短可能直接影响血栓的形成。Ren等[21]房颤射频消融术中抗凝研究共入选511例患者,分为两组。应用心腔内超声发现ACT 250-300s组左房血栓发生率11.2% (33/294) ,而ACT>300s组左房血栓发生率仅为2.8% (6/217 ) ,P < 0.05,提示加强消融过程中抗凝强度可减少血栓发生,均无症状性缺血性卒中事件。
由于目前抗凝策略术前中断华法林,低分子肝素替代治疗,术后继续应用华法林,需要数天才能达到有效抗凝强度,从而增加了血栓栓塞风险。2010年Hussein[22]等在3052例房颤导管消融患者未采用桥接治疗,围手术期继续华法林抗凝,INR平均2.52±0.62,结果缺血性卒中3例,出血性卒中1例,不严重出血34例(1.11%),提示围手术期持续应用华法林安全有效,显著降低血栓栓塞风险。2014年Biase [23]等进行的一项前瞻性多中心研究评估了房颤导管消融围手术期持续应用华法林血栓栓塞的风险。应用华法林组无症状性脑卒中发生率2.1%,停用华法林组无症状性脑卒中发生率高达14.2%,两组间发生率讯在显著差异(P<0.0001)。可见,中断华法林使围手术期血栓风险明显增加(OR:3.8,95% CI),而围手术期持续应用华法林可明显降低缺血性卒中风险。术后是否继续坚持服用华法林目前尚存在争议,一般如无复发,应用华法林至术后2-3个月。
上述研究提示我们房颤导管消融过程中需加强抗凝以减少血栓栓塞发生风险,术中ACT监测保持在300~350s;术前应用华法林者,术中继续应用;术后坚持服用华法林,INR2~3, 如无复发,应用华法林2~3个月。
4.2 导管操作及术式选择策略
心腔内导管操作应轻柔小心,避免鞘内形成血栓、气体进入、充分回抽及持续肝素盐水冲洗。血栓的形成与消融方法及手术中左房内放置导管数量多少有一定关系,即导管数量越多形成血栓栓塞的机会可能就越多。2011年Gaita[24]等一项前瞻性研究连续入选108例行房颤导管消融患者,等数量分为冷冻球囊导管组、盐水灌注导管组和多电极导管组,均对肺静脉进行电隔离,发现无症状脑梗塞比例分别为:5.6%,8.3%,38.9%。显示不同手术器械对房颤导管消融相关无症状脑梗塞有一定关系,并且左房内导管数目越多,形成血栓栓塞的几率在增加。2014年Gui-jian L等研究[25]发现冷冻球囊,盐水灌注导管,激光球囊,多电极导管和nMARQTM(环形多极消融导管)无症状脑梗塞发生率分别为12.5%,13.0%, 17.3%, 27.6%和 32.6%。上述研究显示单一的冷冻球囊消融发生血栓栓塞风险最低,冷盐水灌注导管消融法需要额外应用Lasso环形电极,因此增加了血栓风险,而多电极导管技术血栓栓塞风险最高,同时提示导管数目的增多也增加血栓栓塞的发生。因此,手术过程中应合理选择器械,尽量减少不必要导管的放置和使用。
5 预后
虽然脑梗塞是无临床症状的,但是这些损害累积性效应,可能会成为脑血管事件的一个沉重负担,导致痴呆、记忆缺失的增加,认知功能减低[26-28]。以后有发生症状性脑梗塞的可能,或使其发生症状性脑梗塞的病情加重,并增加脑梗塞复发的危险。
6 小结
我们不能忽视房颤导管消融相关无症状脑梗塞的发生。目前抗凝策略不能完全有效预防无症状脑梗塞,强化导管消融围手术期抗凝,如术中ACT监测保持在300~350s,围手术期不间断应用华法林抗凝等。细化房颤消融方案,合理选择手术器械及方法,以减少脑梗塞发生。
参考文献
1. Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation[J]. Circ Arrhythmia Electrophysiol 2010, 1:3:32-38.
2.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010,303(4):333-340.
3. Piccini JP, Lopes RD, Kong MH, et al. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials[J]. Circ Arrhythm Electrophysiol. 2009, 2(6):626-633.
4. Pappone C1, Vicedomini G, Augello G, et al. Radiofrequency catheter ablation and antiarrhythmic drug therapy: a prospective, randomized, 4-year follow-up trial: the APAF study. Circ Arrhythm Electrophysiol. 2011, 4(6):808-814.
5. Wokhlu A, Monahan KH, Hodge DO, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. Am Coll Cardiol. 2010, 55(21):2308-2316.
6.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009, 2:349–361.
7. Gaita F, Caponi D, Pianelli M, et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation, 2010, 122(17): 1667-1673.
8. Scaglione M, Blandino A, Raimondo C, et al. Impact of ablation catheter irrigation design on silent cerebral embolism after radiofrequency catheter ablation of atrial fibrillation: Results from a pilot study. Journal of cardiovascular electrophysiology, 2012, 23(8): 801-805.
9. Deneke T, Shin D I, Balta O, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm, 2011, 8(11): 1705-1711.
10. Deneke T, Jais P, Scaglione M, et al. Silent Cerebral Events/Lesions Related to Atrial Fibrillation Ablation: A Clinical Review. J Cardiovasc Electrophysiol. 2015, 26(4):455-463.
11. Kalantarian S, Ay H, Gollub RL, et al. Association Between Atrial Fibrillation and Silent Cerebral Infarctions: A Systematic Review and Meta-analysisAtrial Fibrillation and Silent Cerebral Infarctions. Ann Intern Med. 2014, 161(9):650-658.
12. Zhou L, Keane D, Reed G, et al. Thromboembolic complications of cardiac radiofrequency catheter ablation: A review of the reported incidence, pathogenesis and current research directions. J Cardiovasc Electrophysiol 1999, 10:611-620.
13. Ren JF, Marchlinski FE, Callans DJ et al. Left atrial thrombus associated with ablation for atrial fibrillation: Identification with intracardiac echocardiography. J Am Coll Cardiol 2004;10:1861-1867
14.Lickfett L, Hackenbroch M, Lewalter T, et al. Cerebral diffusion-weighted magnetic resonance imaging: a tool to monitor the thrombogenicity of left atrial catheter ablation[J]. Cardiovasc Electrophysiol. 2006, 17(1):1-7.
15. Wieczorek M, Lukat M, Hoeltgen R, et al. Investigation into Causes of Abnormal Cerebral MRI Findings Following PVAC Duty‐Cycled, Phased RF Ablation of Atrial Fibrillation[J]. Journal of cardiovascular electrophysiology, 2013, 24(2): 121-128.
16.Rillig A, Meyerfeldt U, Tilz R R, et al. Incidence and long-term follow-up of silent cerebral lesions after pulmonary vein isolation using a remote robotic navigation system as compared with manual ablation[J]. Circulation: Arrhythmia and Electrophysiology, 2012, 5(1): 15-21.
17. Siklódy C H, Deneke T, Hocini M, et al. Incidence of Asymptomatic Intracranial Embolic Events After Pulmonary Vein Isolation Comparison of Different Atrial Fibrillation Ablation Technologies in a Multicenter Study[J]. Journal of the American College of Cardiology, 2011, 58(7): 681-688.
18. Kilicaslan F, Verma A, Saad E, et al. Transcranial Doppler detection of microembolic signals during pulmonary vein antrum isolation: implications for titration of radiofrequency energy. Cardiovasc Electrophysiol. 2006, 17(5):495-501.
19. Nagy-Balo E, Kiss A, Condie C, et al. Predictors of cerebral microembolization during phased radiofrequency ablation of atrial fibrillation: analysis of biophysical parameters from the ablation generator. Heart Rhythm. 2014, 11(6):977-983.
20. Oh S, Kilicaslan F, Zhang Y, et al. Avoiding microbubbles formation during radiofrequency left atrial ablation versus continuous microbubbles formation and standard radiofrequency ablation protocols: comparison of energy profiles and chronic lesion characteristics[J]. Cardiovasc Electrophysiol. 2006,17(1):72-77.
21. Ren JF, Marchlinski FE, Callans DJ, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast [J]. Cardiovasc Electrophysiol. 2005, 16(5):474-477.
22.Hussein AA, Martin DO, Saliba W,et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm. 2009, 6(10):1425-1429.
23. Biase L, Gaita F, Toso E, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014, 11(5):791-798.
24. Gaita F, Leclercq J F, Schumacher B, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon[J]. Journal of cardiovascular electrophysiology, 2011, 22(9): 961-968.
25. Gui-jian L, Wen-qing Z, Xing-gang W, et al. Association between Ablation Technology and Asymptomatic Cerebral Injury Following Atrial Fibrillation Ablation. Pacing Clin Electrophysiol. 2014.
26. Schwarz N, Kuniss M, Nedelmann M , et al. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm. 2010;7(12):1761-1767.
27. Vermeer S, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003, 348:1215-1222.
28. Knecht S, Oelschlager C, Duning T, et al. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur Heart J. 2008, 29:2125–2132.